Introduction
Lumbar interbody fusion has proven to be an excellent surgical option for the treatment of various spinal conditions such as degenerative disc disease, deformity, infections, trauma, or neoplasms.1 This procedure involves the removal of the intervertebral disc, the preparation of the vertebral endplates and the placement of various types of implants, and its main objective is to restore the intervertebral space and stabilize the treated segment, maintaining adequate height and lordosis.1 Since it was first described by Burns & Capener1 and, later, Briggs & Milligan2, this surgery has been performed mainly through the posterior approach, which involves the dissection of the paraspinal muscles and posterior bone resection to gain access to the disc space.3
In the last 20 years, there has been an emerging and developing interest in anterolateral approaches to the lumbar spine.1,2 These approaches are intended to prevent the occurrence of an injury to the posterior part of the spine and, at the same time, allow exposure of the intervertebral disc. Moreover, they facilitate the placement of larger interbody cages, shorten surgical time in some cases, reduce blood loss, and allow for indirect decompression of the nerve structures.3
The most commonly used lumbar interbody fusion procedures are anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (LLIF) and oblique lumbar interbody fusion (OLIF) or anterior to psoas interbody fusion (ATP).1,3 Although they have similar surgical objectives, they differ in terms of the type of patients for whom they are indicated, surgical planning, surgical technique, as well as their potential risks and complications.4
In view of the above, the aim of this study was to describe the clinical outcomes of the use of OLIF for the treatment of degenerative disc disease in a university hospital in Spain.
Materials and methods
Study type
Case series study.
Sample
Using consecutive sampling, all patients with radiological findings of degenerative disc disease and clinical signs of low back pain, lumbar radiculopathy and/or neurogenic claudication in whom OLIF was performed in a university hospital in Ourense (Spain) between January 2018 and June 2020 were included (n=32). All patients included were treated at 1, 2 or 3 spine segments, and the minimum follow-up period was 1 year.
Data collection and variables
The following patient data were collected retrospectively upon reviewing their medical records: age, sex, spine segment in which fusion was performed, number of spine segments treated, surgical time, and length of hospital stay. Clinical evolution was assessed using the Oswestry Disability Index (ODI), which was administered to the patient prior to surgery and 12 months after surgery. Likewise, lumbar and radicular pain was evaluated by means of the visual analog scale (VAS) before the surgery and 12 months after the intervention. In addition, peri- and postoperative complications were documented.
Surgical technique
Patient positioning
The left retroperitoneal approach was performed in all patients included in the present case series. To this end, the patient is placed in right lateral decubitus with the left side more elevated and two straight supports are placed, one in the interscapular region and the other in the coccyx, leaving the abdomen free. Then, all pressure points are checked, and the patient’s trunk is stabilized with a wide adhesive cloth fixed to the surgical table. Also, a flat pillow is placed between both lower extremities, positioning the right leg flexed, for greater stability, and the left leg slightly flexed, and two other strips of adhesive tape are glued across the lower extremities.
The upper extremities are flexed at 90° and pads are placed in both armpits, while the head is placed in a neutral position on a pillow. Furthermore, before starting the approach, it should be confirmed that there is an adequate radiological image of the anteroposterior and lateral views and that it is possible to mobilize the fluoroscopic device without obstacles (Figure 1A).
Retroperitoneal approach
Once the patient is in the surgical position described in the previous section, the projection of the disc spaces to be surgically treated is marked on the patient’s skin. Then, an incision is made 3 to 5 centimeters (cm) from the front of this mark. When several disc spaces must be treated, marks are made on the discs to be fused and an oblique incision is made to allow access to all discs (Figure 1B).
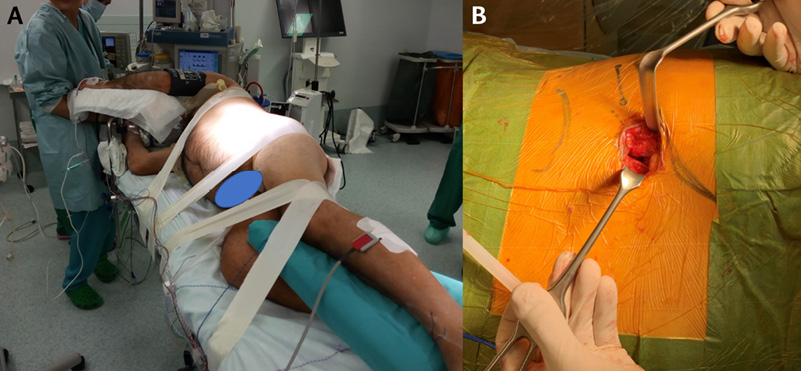
Figure 1. Surgical treatment of degenerative disc disease with oblique lumbar interbody fusion. 1A. Patient in right lateral decubitus position. 1B. Left retroperitoneal approach. The marks made on the twelfth rib and the iliac crest are observed, as well as the projection of the disc space to be treated.
Source: Image obtained while conducting the study.
To treat degenerative disc disease in a single disc space, a 3- to 4-cm incision is usually sufficient (Figure 2A). Once the incision is made, the subcutaneous tissue is opened with a monopolar scalpel. Next, the superficial aponeurosis of the external oblique is identified, and its fibers are separated longitudinally with a cold scalpel or scissors (Figure 2B). Subsequently, the same procedure is performed on the external part of the internal oblique and transversus abdominis (Figure 2C and 2D). It should be noted that throughout the procedure, it is important to ensure a good longitudinal dissection of these muscles, as this will facilitate the approach, especially in patients with several affected spine segments.
The transversalis fascia will be observed under the transversus abdominis; since it is sometimes very thin and transparent, the fat of the retroperitoneum must be identified (Figure 2E). Once this space is reached, a blunt dissection is performed with a finger or cotton swab, initially from posterior to anterior position and in a craniocaudal direction to separate the organs contained in the peritoneal cavity from the surgical approach zone. Then, the psoas muscle is palpated, and the hand is slid anteriorly until it touches the anterolateral part of the vertebral body (Figure 2F). Afterwards, the disc space is marked with the help of the retractors.
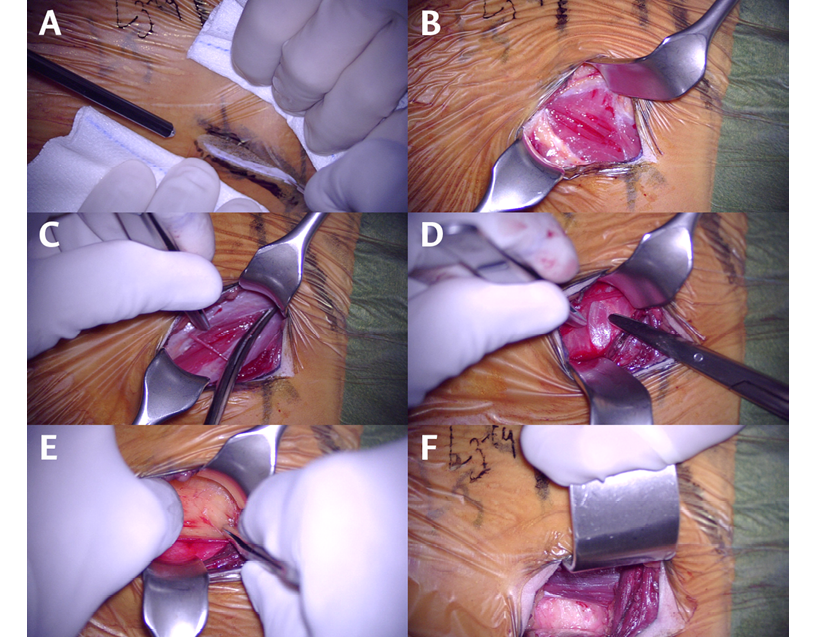
Figure 2. Surgical approach. 2A. Skin incision. 2B. Opened external oblique fascia. 2C. Dissection of the internal oblique. 2D. Identification of the transversus abdominis muscle. 2E. Entry into the retroperitoneum. 2F. Psoas muscle and vertebral prepsoas space.
Source: Image obtained while conducting the study.
Diskectomy and implant placement
After radiologically verifying that the spine segment is the correct one, the surgical retractor is placed definitively. Usually, two retractors are used for separation, one in the cranial direction and the other in the caudal direction. The organs contained in the peritoneal cavity are usually separated from the surgical field by gravity and do not need to be retracted. In addition, the psoas muscle should be partially separated from the spine using a wide Cobb dissector. Usually, diskectomy is started in the middle of the vertebral disc, verifying by means of fluoroscopy (lateral view) that the anatomical location where the surgery will be started is correct (Figure 3A). As the diskectomy progresses, the trajectory to be used for the procedure (dissection, diskectomy and implant placement) should be corrected, ensuring that a lateral trajectory is maintained within the disk space, i.e., perpendicular to the operating room floor, rather than an oblique trajectory. Subsequently, the contralateral side of the vertebral disc must be checked once again by means of X-rays (lateral and anteroposterior views) to ensure that the contralateral side of the vertebral disc has been reached without going beyond the posterior wall of the vertebra.
After completing the diskectomy and preparation of the vertebral endplates, the size of the final implant is measured (Figure 3B-C). Ideally, the implant should reach the edges of the vertebra, settling on the annular epiphysis. The height and degree of lordosis will depend on the type of degenerative spinal disease being treated. Demineralized bone matrix is used to fill the interbody cage, except in those cases where there is a risk of pseudarthrosis or when an iliac crest graft obtained through the same incision is used.
After placing the interbody implant, surgical hemostasis should be checked. It is worth mentioning that postoperative drainage is not usually placed. Finally, the aponeurosis of the oblique muscles is closed with loose subcutaneous stitches, while the skin is closed with staples.
Posterior fixation of the spine
In all patients in this case series, the interbody fusion was completed with posterior fixation with transpedicular screws. Once the abdominal incision was closed, the patient was placed in prone position and screws were placed percutaneously (Figure 3D).
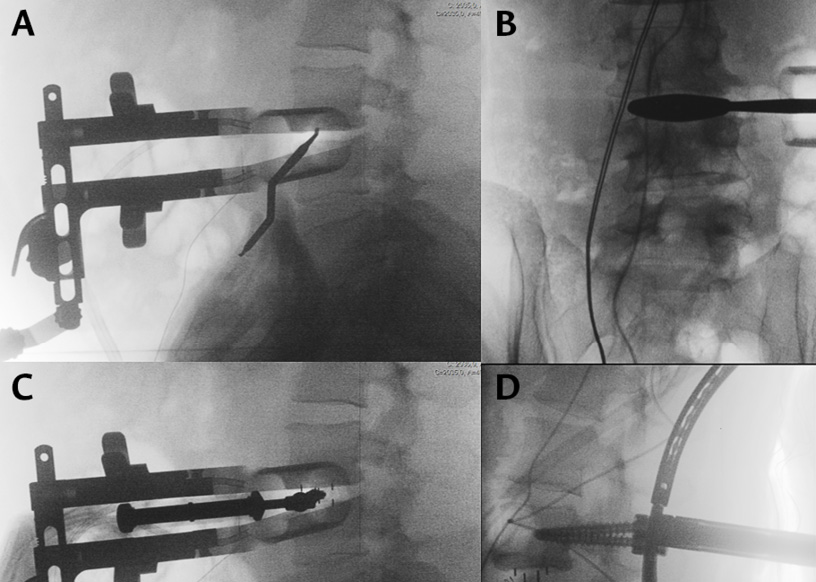
Figure 3. Intraoperative X-ray images. 3A. Dissector marking of the middle of the vertebral disc.
3B. Calculation of the definitive implant size by testing after diskectomy. 3C. Placement of the interbody cage. 3D. Pedicle screw placement with the usual percutaneous technique.
Source: Image obtained while conducting the study.
For interbody fusion, a Clysdale™ Spinal System box (Medtronic Sofamor Danek, Minneapolis, USA) was used, while the CD Horizon Sextant™ II and CD Horizon Longitude™ II systems (Medtronic Sofamor Danek, Minneapolis, USA) were used for percutaneous transpedicular screw fixation.
On the day of surgery, all patients remained on bed rest, with free mobility, and pharmacological pain treatment was started after 8 hours. On the first postoperative day, patients were allowed to sit up and begin ambulation. Lumbar orthoses were not used after the fusion surgeries. If the patient could ambulate and postoperative pain could be controlled with oral medication, the patient was discharged 48 hours after surgery.
Statistical analysis
All analyses were performed using the SPSS (version 26) and R software. Data are described using frequencies and percentages for categorical variables, and means, standard deviations (SD) and ranges (minimum value-maximum value) for quantitative variables, since the data had a normal distribution (Shapiro-Wilk test). Bivariate analyses were performed to compare the ODIs and VAS scores of low back and radicular pain identified before surgery and at 12-month follow-up. Comparisons between quantitative variables were made using the Mann-Whitney test, while the Chi-square test was used for comparisons between qualitative variables. A statistical significance value of p<0.005 was considered in all analyses.
Ethical considerations
This research followed the ethical principles for the conduct of biomedical studies involving human subjects established in the Declaration of Helsinki.5 In addition, the study was approved by the Health Care and Research Ethics Committee of the Complexo Hospitalario Universitario de Ourense by means of minutes 20/013 of 2021.
Results
Of the 32 patients operated on during the study period, 56.25% (n=18) were women and the mean age was 56 years (SD±14.53; range: 30-79 years). Regarding the type of degenerative disc disease, 19 cases (59.38%) involved spinal or foraminal stenosis without spondylolisthesis. The number of fused spine segments was 3, 2 and 1 in 9.38%, 12.5% and 78.1% of individuals, respectively, so the total number of interbody implants used was 42. The most frequently operated spine fusion segment was L4-L5 (50%; n=21), followed by L3-L4 (28.57%; n=12), and L2-L3 (16.67%; n=7). Finally, the mean operative time and length of hospital stay were 153.13 minutes (SD±41.2; range: 88-210 minutes) and 2.53 days (SD±1.72; range: 1-5 days), respectively. The clinical and sociodemographic characteristics of the patients are presented in Table 1.
Table 1. Demographic and clinical characteristics of patients with lumbar degenerative disc disease included in the study (n=32).
|
Variable |
n (%) |
|
Age (years) - mean (SD) |
56.21 (SD ± 14.53) |
|
Intervention duration (minutes) - mean (SD) |
153.3 (SD ± 41.2) |
|
Hospital stay (days) - mean (SD) |
2.53 (SD ± 1.72) |
|
Sex |
|
|
Male |
14 (43.75%) |
|
Female |
18 (56.25%) |
|
Type of degenerative disc disease |
|
|
Degenerative spondylolisthesis |
13 (40.62%) |
|
Foraminal or spinal stenosis |
19 (59.38%) |
|
Number of segments treated |
|
|
1 segment |
25 (78.1%) |
|
2 segments |
4 (12.5%) |
|
3 segments |
3 (9.38%) |
|
Spine segment |
|
|
L1-L2 |
2 (4.76% |
|
L2-L3 |
7 (16.67%) |
|
L3-L4 |
12 (28.57%) |
|
L4-L5 |
21 (50%) |
SD: standard deviation.
Source: Own elaboration.
The average ODI was 52.3 (SD±4.96) preoperatively and 12.3 (SD±3.19) one year after the procedure, showing a significant improvement (p<0.001). On the other hand, improvement was identified both in the mean VAS score of low back pain (preoperative: 8.81; SD±0.62 versus one year later: 2.12; SD±0.89; p=0.002), and in the VAS score of radicular pain (preoperative: 6.79; SD±3.41 versus one year later: 1.53; SD±2.98; p<0.001).
Regarding postoperative complications, 12.5% (n=4) of the patients had psoas muscle weakness when flexing the hip, which in no case persisted for more than 2 weeks after surgery; 3.1% (n=1) presented symptoms of sympathetic nervous system injury (differences in extremity temperature and swelling), which resolved progressively within 3 months after surgery; and 9.375% (n=3) reported sensory alterations in the groin and/or thigh, which also resolved progressively in the first weeks after surgery. Finally, radiological follow-up identified implant subsidence in only 1 patient (3.1%), who did not show clinical manifestations (Table 2).
Table 2. Postoperative evolution and presence of complications in the patients included in the study (n=32).
|
Variable |
Preoperative Mean (SD) |
Postoperative Mean (SD) |
p-value |
|
Oswestry Disability Index |
52.3 (±4.96) |
12.3 (±3.19 |
< 0.001 |
|
Low back pain VAS score |
8.81 (±0.62) |
2.12 (±0.89) |
0.002 |
|
VAS score of radicular pain |
6.79 (±3.41) |
1.53 (±2.98)) |
< 0.001 |
|
Post-surgical complications - n (%) |
|||
|
Weakness in hip flexion (psoas muscle) |
4 (12.5%) |
||
|
Sympathetic nervous system injury |
1 (3.1%) |
||
|
Sensory alterations |
3 (9.38%) |
||
|
Intervention duration - mean (SD) |
153.13 (±41.2) |
||
SD: standard deviation. VAS: visual analog scale.
Source: Own elaboration.
Discussion
Degenerative disc disease in lumbar facet discs is common in older adults and is one of the main causes of disability.1,4 Lumbar spondyloarthrosis can cause mechanical or radicular pain, signs and symptoms of claudication, loss of mobility, and decreased quality of life.6 Interbody fusion of the spine at the affected segments is a surgical option to stabilize the painful mobile segment of the spine, and its use can achieve an indirect decompression of the neural elements, restore lordosis and correct the deformity.7
In the 1930s, Burns & Capener1 described the first interbody fusion in the treatment of spondylolisthesis by anterior approach. However, the first description of PLIF was made by Briggs & Milligan2 in 1944 and developed by Cloward6 in the 1950s. In turn, Harms & Rolinger8 introduced TLIF as an alternative to PLIF in 1982. In addition, following the first description of ALIF as a treatment for Pott disease in the 1930s, this technique has been extensively studied and frequently used in the treatment of degenerative lumbar spine disease.6 However, its disadvantages are the limitation in the spine segments that can be treated (L5-S1) and the risk of vascular injury and injury to the organs contained in the peritoneal cavity, as well as retrograde ejaculation.9
Ozgur et al.10 were the first to describe LLIF in 2001, a surgical procedure in which access to the spine is sought through the retroperitoneum to take advantage of it, and the approach is made through the psoas muscle. Subsequently, these authors modified this technique in order to perform it in a less invasive manner and called it extreme lateral interbody fusion (XLIF). In this regard, it should be noted that the main disadvantages of this approach are the limitation to access L5-S1 and, in some patients, L4-L5, as well as the requirement of intraoperative neurophysiological monitoring and the risk of injury to the lumbar plexus.11
The technique of anterior oblique lumbar interbody fusion to the psoas muscle was first described in 1997 by Mayer,12 although the term OLIF was coined in 2012 by Silvestre et al.13 OLIF is an alternative to LLIF, since it is not necessary to go through the psoas muscle and the intervertebral disc is accessed through the space between this muscle and the great vessels in order to reduce the risk of muscle and lumbar plexus injury, which makes it possible to avoid intraoperative neurophysiological monitoring.14,15 As in LLIF, in OLIF it is not necessary to perform laminectomy or facetectomy via the posterior approach or disinsertion of the paraspinal muscles.14,15
Another advantage of OLIF is access to the L4-L5 disc space, which can be complex in men with high iliac crest and bulky psoas muscle. Furthermore, the anterior approach to the psoas muscle allows access to the L5-S1 disc from the lateral position; however, the proximity of the left iliac vein must be considered.16 This study only presents cases treated by the OLIF approach in which the L5-S1 disc was not intervened, since the surgical technique and the type of implant used differ from those used in the rest of the lumbar spine segments.
Lateral lumbar interbody fusion techniques, both LLIF and OLIF, minimize soft tissue injury and reduce the length of hospital stay and intraoperative blood loss, while matching or improving the clinical and radiological results of posterior techniques.17,18 Deformity in the coronal or sagittal planes of the vertebrae can be corrected by using larger interbody cages and different lordosis angles.19 Additionally, these techniques have been shown to increase the height of the foramen and the surface of the spinal canal, achieving an indirect decompression of the nerve structures20 (Figure 4A-D).
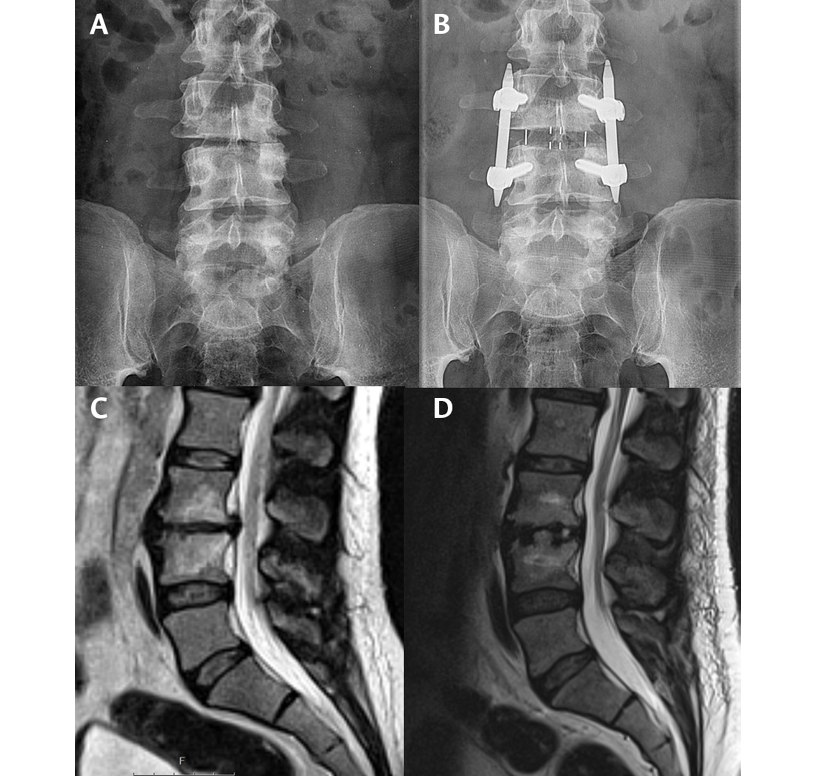
Figure 4. X-ray and nuclear magnetic resonance imaging of the lumbar spine. 4A. Preoperative X-ray. 4B. Postoperative X-ray showing the restoration of the disc space. 4C. Preoperative magnetic resonance imaging. 4D. Postoperative nuclear magnetic resonance imaging showing restoration of disc height and retraction of disc protrusion.
Source: Image obtained while conducting the study.
The OLIF technique is suitable for treating degenerative diseases that require the restoration of disc height. For this reason, it is very useful in the treatment of patients with degenerative spondylolisthesis or scoliosis.21 Moreover, the OLIF technique is being used more frequently in patients with adjacent segment disease or postlaminectomy syndrome, in whom conventional surgery has a high risk of durotomy.22
The most frequent complications of the OLIF technique described in the literature are incisional pain, symptoms of injury to the sympathetic nervous system in the lower extremities, weakness of the psoas muscle, and vascular lesions.23 In this case series, psoas muscle weakness was the most common complication in 12.5% of cases (4/32 patients), although it did not persist for more than 2 weeks in these patients.
To minimize this type of complications, it is advisable to adequately review the preoperative images to assess the anatomical space anterior to the psoas muscle, as well as the vertebral and vascular anatomy.14,24 Progressive dissection of the abdominal wall planes, using blunt dissection and directly visualizing the anatomical structures, avoids injury to the subcostal, ilioinguinal, iliohypogastric and lateral femoral cutaneous nerves.25 Once the retroperitoneum is accessed, it is recommended to continue the blunt dissection by performing posteroanterior and caudal-cranial movements until the vertebral space in front of the psoas muscle is adequately located.23 The space anterior to the psoas muscle can be enlarged with a slight retraction or posterior dissection of the anterior belly of the psoas muscle.26 However, it should be noted that prolonged retraction of the psoas against the transverse processes may cause lumbar plexus injuries. On the other hand, meta-analyses have reported that the risk of ipsilateral hip flexion weakness, transient thigh pain and lumbar plexus injury are lower in OLIF than in LLIF.14,27 Conversely, there is an increased risk of vascular or sympathetic nervous system injury in OLIF.14,28
There is no consensus on the need to complement oblique interbody fusion with a posterior fixation system.29 Most studies reviewed during the course of this study support its use by claiming a decrease in the rates of pseudarthrosis or implant subsidence.30 In this case series, the interbody cages used were made of polyether ether ketone and did not have complementary fixation or screw systems, so all patients received posterior fixation with percutaneous transpedicular screws at another surgical time. Currently, interbody cages with integrated fixation systems are available, but further studies are required to demonstrate that they could have adequate fusion rates without the need for posterior support.
Another area of debate today is whether to perform the entire procedure in a single position or in different stages.31 At the medical institution where the present study was performed, the surgery was done in two stages, initially in lateral decubitus and then in prone position for posterior fixation. In this regard, it is considered that this does not prolong surgical times excessively, nor does it imply excessive work for the medical team.
One of the limitations of the present study is the follow-up period of 12 months. Although it is considered sufficient to collect most of the relevant clinical results both perioperatively and postoperatively, complications such as the development of adjacent segment disease or the evaluation of the impact on long-term spinopelvic parameters require studies with longer follow-up periods.32,33
Conclusions
The oblique approach for lumbar interbody fusion is a viable option among the different spinal fusion techniques. In the present study, its complication rate was low, and it resulted in improvement in terms of pain and disability. Knowledge of the anatomy of the abdominal wall and retroperitoneum, progressive dissection of the psoas muscle when necessary, and adequate preparation of the disc space are fundamental steps to obtain a good surgical outcome.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.de Kunder SL, Rijkers K, Caelers IJMH, de Bie RA, Koehler PJ, van Santbrink H. Lumbar Interbody Fusion: A Historical Overview and a Future Perspective. Spine (Phila Pa 1976). 2018;43(16):1161-8. https://doi.org/gjv675.
2.Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J. Bone Jt. Surg. 1944;26(1):125-130.
3.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(Suppl. 26):S331-7. https://doi.org/cff9vn.
4.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015;1(1):2-18. https://doi.org/ghqpn6.
5.World Medical Association (WMA). WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects. Fortaleza: 64th WMA General Assembly; 2013.
6.Reid PC, Morr S, Kaiser MG. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J. Neurosurg. Spine. 2019;31(1):1-14. https://doi.org/gjrtbc.
7.Derman PB, Albert TJ. Interbody Fusion Techniques in the Surgical Management of Degenerative Lumbar Spondylolisthesis. Curr. Rev. Musculoskelet Med. 2017;10(4): 530-8. https://doi.org/gjr94n.
8.Harms J, Rolinger H. Die operative Behandlung der Spondylolisthese durch dorsale Aufrichtung und ventrale Verblockung. Orthop Ihre Grenzgeb. 1982;120(3):343-7. https://doi.org/b46jts.
9.Mobbs RJ, Lennox A, Ho YT, Phan K, Choy WJ. L5/S1 anterior lumbar interbody fusion technique. J Spine Surg. 2017;3(3):429-32. https://doi.org/kx7m.
10.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-43. https://doi.org/frv8vb.
11.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011; 36(1):26-32. https://doi.org/btjqmm.
12.Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1997;22(6):691-700. https://doi.org/dqt57k.
13.Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J. 2012;6(2):89-97. https://doi.org/gb94wh.
14.Spiessberger A, Arvind V, Dietz N, Grueter B, Huber F, Guggenberger R, et al. A Comparison of Complications and Clinical and Radiologic Outcome Between the Mini-open Prepsoas and Mini-open Transpsoas Approaches for Lumbar Interbody Fusion: A Meta-Analysis. Clin Spine Surg. 2020;33:271-9. https://doi.org/kz2q.
15.Kim JS, Choi WS, Sung JH. Minimally Invasive Oblique Lateral Interbody Fusion for L4-5: Clinical Outcomes and Perioperative Complications. Neurosurg. 2016;63:190-1. https://doi.org/kz2s.
16.DiGiorgio AM, Edwards CS, Virk MS, Chou D. Lateral Prepsoas (Oblique) Approach Nuances. Neurosurg Clin N Am. 2018;29(3):419-426. https://doi.org/gdzpkz.
17.Nakashima H, Kanemura T, Satake K, Ishikawa Y, Ouchida J, Segi N, et al. Comparative Radiographic Outcomes of Lateral and Posterior Lumbar Interbody Fusion in the Treatment of Degenerative Lumbar Kyphosis. Asian Spine J. 2019;13(3):395-402. https://doi.org/kz2t.
18.Iwamae M, Matsumura A, Namikawa T, Kato M, Hori Y, Yabu A, et al. Surgical Outcomes of Multilevel Posterior Lumbar Interbody Fusion versus Lateral Lumbar Interbody Fusion for the Correction of Adult Spinal Deformity: A Comparative Clinical Study. Asian Spine J. 2020;14(4):421-29. https://doi.org/kz2v.
19.Rabau O, Navarro-Ramirez R, Aziz M, Teles A, Mengxiao Ge S, Quillo-Olvera J, et al. Lateral Lumbar Interbody Fusion (LLIF): An Update. Global Spine J. 2020;10(Suppl 2):17S-21S. https://doi.org/kz2x.
20.Limthongkul W, Tanasansomboon T, Yingsakmongkol W, Tanaviriyachai T, Radcliff K, Singhatanadgige W. Indirect Decompression Effect to Central Canal and Ligamentum Flavum After Extreme Lateral Lumbar Interbody Fusion and Oblique Lumbar Interbody Fusion. Spine (Phila Pa 1976). 2020;45(17): 1077-84. https://doi.org/kz2z.
21.McGowan JE, Kanter AS. Lateral Approaches for the Surgical Treatment of Lumbar Spondylolisthesis. Neurosurg Clin N Am. 2019;30(3):313-322. https://doi.org/kz22.
22.Kudo Y, Okano I, Toyone T, Matsuoka A, Maruyama H, Yamamura R, et al. Lateral lumbar interbody fusion in revision surgery for restenosis after posterior decompression. Neurosurg Focus. 2020;49(3): E11.
https://doi.org/kz23.
23.Quillo-Olvera J, Lin GX, Jo HJ, Kim JS. Complications on minimally invasive oblique lumbar interbody fusion at L2-L5 levels: a review of the literature and surgical strategies. Ann Transl Med. 2018;6(6):101. https://doi.org/gdgcvz.
24.Li JX, Phan K, Mobbs R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg. 2017;98:113-123. https://doi.org/f9xdjs.
25.Kanemura T, Satake K, Nakashima H, Segi N, Ouchida J, Yamaguchi H, et al. Understanding Retroperitoneal Anatomy for Lateral Approach Spine Surgery. Spine Surg Relat Res. 2017; 1(3):107-20. https://doi.org/gnq2wr.
26.Davis TT, Hynes RA, Fung DA, Spann SW, MacMillan M, Kwon B, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine. 2014;21(5):785-93. https://doi.org/f6mp3s.
27.Kim WJ, Lee JW, Kim SM, Park KY, Chang SH, Song DG, et al. Precautions for Combined Anterior and Posterior Long-Level Fusion for Adult Spinal Deformity: Perioperative Surgical Complications Related to the Anterior Procedure (Oblique Lumbar Interbody Fusion). Asian Spine J. 2019;13(5): 823-31. https://doi.org/kz7g.
28.Walker CT, Farber SH, Cole TS, Xu DS, Godzik J, Whiting AC, et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine. 2019;30(4):1-15. https://doi.org/kz24.
29.He W, He D, Sun Y, Xing Y, Wen J, Wang W, et al. Standalone oblique lateral interbody fusion vs. combined with percutaneous pedicle screw in spondylolisthesis. BMC Musculoskelet Disord. 2020;21(1):184. https://doi.org/gjpcq9.
30.Zeng ZY, Xu ZW, He DW, Zhao X, Ma WH, Ni WF, et al. Complications and Prevention Strategies of Oblique Lateral Interbody Fusion Technique. Orthop Surg. 2018;10(2):98-106. https://doi.org/gdn8ps.
31.Buckland AJ, Ashayeri K, Leon C, Manning J, Eisen L, Medley M, et al. Single position circumferential fusion improves operative efficiency, reduces complications and length of stay compared with traditional circumferential fusion. Spine J. 2021;21(5):810-20. https://doi.org/kz25.
32.Li GQ, Tong T, Wang LF. Comparative analysis of the effects of OLIF and TLIF on adjacent segments after treatment of L4 degenerative lumbar spondylolisthesis. J Orthop Surg Res. 2022;17(1):203. https://doi.org/kz26.
33.Li R, Shao X, Li X, Liu Y, Jiang W. Comparison of clinical outcomes and spino-pelvic sagittal balance in degenerative lumbar spondylolisthesis: Minimally invasive oblique lumbar interbody fusion (OLIF) versus transforaminal lumbar interbody fusion (TLIF). Medicine (Baltimore). 2021;100(3):e23783. https://doi.org/kz27.