review article
Developmental dysplasia of the hip may predispose to osteoarthritis: From inflammation to destruction, a literature review
La displasia del desarrollo de la cadera puede predisponer a osteoartritis,
de la inflamación a la destrucción: revisión de la literatura
Jorge Alvarado-García1 Liliana García-Ortiz2
Liliana García-Ortiz2 José Gutiérrez-Salinas3
José Gutiérrez-Salinas3 Tania Jazmín Ruiz-Sánchez4
Tania Jazmín Ruiz-Sánchez4
1 Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Hospital Regional de Puebla, Trauma and Orthopedics Service, Puebla, Mexico.
2 Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Centro Médico Nacional 20 de Noviembre, Genomic Medicine Division, Mexico City, Mexico.
3 Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Centro Médico Nacional 20 de Noviembre, Biomedical Research Division, Biochemistry and Experimental Medicine Laboratory, Ciudad de México, México.
4 Universidad Nacional Autónoma de México, Cuautitlán School of Higher Education, Undergraduate Program in Diagnostic Biochemistry, State of Mexico, Mexico.
Open access
Received: 17/04/2023
Accepted: 4/05/2023
Corresponding author: Liliana García Ortiz. División de Medicina Genómica, Centro Médico Nacional 20 de Noviembre, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Ciudad de México, México. Email: garortiz@yahoo.com.
How to cite: Alvarado-García J, García-Ortiz L, Gutiérrez-Salinas J, Ruiz-Sánchez J. Developmental dysplasia of the hip may predispose to osteoarthritis: From inflammation to destruction. A literature review. Rev Col Or Tra. 2023;37(2):e19. English. doi: https://doi.org/10.58814/01208845.19
Cómo citar: Alvarado-García J, García-Ortiz L, Gutiérrez-Salinas J, Ruiz-Sánchez J. [La displasia del desarrollo de la cadera puede predisponer a osteoartritis: de la inflamación a la destrucción. Revisión de la literatura]. Rev Col Or Tra. 2023;37(2):e19. English. doi: https://doi.org/10.58814/01208845.19
Copyright: ©2023 Sociedad Colombiana de Cirugía Ortopédica y Traumatología. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, as long as the original author and source are credited.
Abstract
Introduction: Developmental dysplasia of the hip (DDH) encompasses a set of abnormalities related to the maturation process of the acetabulum and the proximal third of the femur. If not treated properly and promptly, patients with this condition may eventually develop osteoarthritis (OA).
Objective: To compile and synthesize scientific evidence published between January 2000 and February 2023 on the pathophysiology of DDH and its relationship to the development of hip OA in terms of genetic, inflammatory and immunological pathophysiological mechanisms.
Methodology: A literature review was performed in biomedical literature databases (PubMed/Medline, Embase, SciELO) and bioinformatic resources (e-Ensambl, STRING), using terms such as "hip dysplasia", "osteoarthritis", "etiology", and "genes". Clinical and genetic observational studies involving human subjects were included.
Results: The initial search yielded 349 records, of which 23 met the eligibility criteria. Genes that interact with genetic modules may play a role in the development of joints and the etiology of diseases that affect the bones and cartilage; however, the mechanical instability caused by DDH activates inflammatory and immunological factors, predisposing to OA. Based on the information obtained, it is possible to consider that there is a very close relationship between DDH and OA.
Conclusions: Knowing the genetic, inflammatory and immunological pathophysiological mechanisms of DDH and OA favors timely diagnosis and, consequently, allows providing proper treatment to reduce and control long-term damage and improve the patient's quality of life.
Keywords: Development Dysplasia of the Hip; Osteoarthritis; Genes; Inflammation; Immunology (MeSH).
Resumen
Introducción. La displasia del desarrollo de la cadera (DDC) abarca un conjunto de anormalidades relacionadas con el proceso de maduración del acetábulo y del tercio proximal del fémur Si no se trata de manera adecuada y oportuna, los pacientes con esta condición pueden desarrollar osteoartritis (OA) eventualmente.
Objetivo. Recopilar y sintetizar evidencia científica publicada entre enero de 2000 y febrero de 2023 sobre la fisiopatología de la DDC y su relación con el desarrollo de OA de cadera en términos de los mecanismos fisiopatológicos genéticos, inflamatorios e inmunológicos.
Materiales y métodos. Se realizó una revisión de la literatura en bases de datos de literatura biomédica (PubMed/Medline, Embase, SciELO) y herramientas bioinformáticas (e-Ensambl, STRING), mediante términos como “displasia de cadera”, “osteoartritis”, “etiología” y “genes”. Se incluyeron estudios observacionales clínicos y genéticos realizados en humanos.
Resultados. La búsqueda inicial arrojó 349 registros, de los cuales 23 cumplieron los criterios de elegibilidad. Los genes que interactúan con módulos genéticos parecen participar en el desarrollo articular y la etiología de las enfermedades relacionadas con el cartílago y el hueso; sin embargo, la inestabilidad mecánica producida por la DCC activa factores inflamatorios e inmunológicos, predisponiendo OA. A partir de la información encontrada, se puede considerar que existe una relación muy estrecha entre DDC y OA.
Conclusiones. Conocer los mecanismos fisiopatológicos genéticos, inflamatorios e inmunológicos de DDC y OA favorece la realización de un diagnóstico oportuno y, en consecuencia, posibilita brindar un tratamiento adecuado para disminuir y controlar el daño a largo plazo y mejorar la calidad de vida del paciente.
Palabras clave: Displasia del desarrollo de la cadera; Osteoartritis; Genes, Inflamación; Inmunología (DeCS).
Introduction
Developmental dysplasia of the hip (DDH) refers to a group of abnormalities related to the maturation process of the acetabulum and proximal third of the femur, which can occur during embryological, fetal, or infancy stages.1,2 These alterations range from hip instability and acetabular and/or femoral head dysplasia to subluxation or dislocation of the femoral head.1,2 If DDH is not properly diagnosed and corrected, over time it leads to osteoarthritis (OA), a degenerative disease characterized by articular cartilage damage, osteophyte formation, and joint deformity that manifests itself in gait disturbances (such as range limitation) and chronic mechanical pain.1,3,4
At present, the etiology of DDH is unknown. However, several related risk factors have been described, including mechanical (breech presentation at birth, oligohydramnios, ligamentous hyperlaxity, lower limb deformity, or left side involvement of the baby in the womb), hereditary (first-degree relatives and first-born children with DDH, or multiple pregnancy), demographic (female sex), and genetic factors, such as the presence of variants, regulatory elements, and enhancers relative to the promoter of the growth differentiation factor 5 (GDF5) gene, which encodes for a ligand associated with the transforming growth factor beta family (TGF-β). These ligands can bind to TGF-β receptors to recruit and activate transcription factors called SMAD, which regulate gene expression, thus participating in normal bone and joint development, as they can initiate chondrogenesis and osteogenesis through epiphyseal cartilage proliferation. In this regard, it has been reported that mutations in the GDF5 gene are associated with various types of dysplasia and synostoses, as well as with an individual’s susceptibility to OA.1,5-7
The prevalence of hip OA ranges from 0.37% to 27% depending on age, sex, place of origin, and degree of involvement. In this regard, the highest prevalence of hip OA has been described in North America and the lowest in Latin America (Table 1).8-12
Table 1. Prevalence and age of occurrence of osteoarthritis of the hip reported in the literature.8-12
|
Prevalence of hip osteoarthritis
|
Age (in years)
|
Place of origin
|
Reference
|
|
27%
|
45
|
United States
|
Lespasio MJ et al.8
|
|
7 - 25 %
|
55
|
Europe
|
Lievense AM. et al.9
|
|
9.6% - 18%
|
60
|
World
|
Leung Y. et al.10
|
|
6.8%
|
65
|
Asia
|
Fransen M. et al.11
|
|
0.37% - 26.5%
|
18-40
|
Latin America
|
Andrade et al.12
|
Source: Own elaboration.
Several clinical and genetic research trials and studies have found a correlation between DDH and OA.13-16 For example, Terjesen13 described that pediatric patients with DDH and subluxation had an increased risk factor for developing hip OA when reconstructive surgery was performed late. According to Terjesen,13 the study conducted by Wiberg in 17 hips with acetabular dysplasia or subluxation identified an association between age and degree of severity of hip OA, as the mean ages at diagnosis of OA in women were 41 years in patients with subluxation and 53 years in those with acetabular dysplasia without subluxation. In addition, Mabuchi et al.16 found that 8 patients in 4 generations of a Japanese family presented with acetabular dysplasia during adolescence and hip OA at ∼40 years, suggesting autosomal dominant inheritance.
On the other hand, according to Hatzikotoulas et al,6 although there is no direct relationship between DDH and OA, genome-wide association studies (GWAS) have shown that these two diseases share genes such as the GDF5 gene and genes encoding for some cytokines and proinflammatory cytokines.
Experimental studies in mice have identified that cell density during joint formation is increased at the site of the future joint, which is known as the interzone. The cells in the interzone are characterized by being GDF5 positive and by retaining an uninterrupted spatiotemporal flow in order to participate in joint development. Thus, experimental observations of GDF5 in interzone cells that are related to joint regeneration may be a starting point to favor its clinical application. However, it should be noted that it has been described that these cells naturally present a limited regenerative capacity, so further studies are needed to demonstrate the regenerative mechanisms in OA.17
Furthermore, it has been observed that hip OA begins with cartilage, bone and/or synovial tissue damage, is caused by a series of microtraumas secondary to biomechanical instability of the joint (hip subluxation and/or dislocation), and is favored by genetic, immunological and inflammatory mechanisms that together contribute to the formation of a microenvironment of regulatory elements, which will lead to the destruction of the hip joint in the short or medium term.16,17
With the above in mind, the objective of this narrative literature review was to compile and synthesize scientific evidence published between January 2000 and February 2023 on the pathophysiology of DDH and its relationship to the development of hip OA in terms of genetic, inflammatory, and immunological pathophysiological mechanisms.
Methodology
A search for articles on the subject was performed in the PubMed/Medline, SciELO and Embase databases, while a search for studies on specific genes that were previously identified in the biomedical literature was conducted in the e-Ensambl and STRING bioinformatics tools.
One of the authors conducted the article search, while a second author reviewed the articles for final inclusion; both processes were carried out independently, randomly, and freely. The following search strategy was used: study types: clinical or genetic studies, case reports, observational studies, and systematic reviews; period of publication: January 2000 and February 2023; language of publication: Spanish or English; search terms: “congenital hip dysplasia”, “hip”, “osteoarthritis”, “etiology”, “genetics factors”, “genes”, “gene expression”, “GWAS”, “proteomic”. Studies involving human subjects and research using experimental models were included.
It is worth mentioning that all articles that were not open access or did not have their full text available through the institution’s library system were excluded, as were articles with a different type of study than those mentioned above, written in a language other than Spanish or English, and those that did not address the review’s topic of interest (for example, research on osteoarthritis in a region other than the hip or on genes unrelated to the disease), or were conducted in the pediatric population.
Results
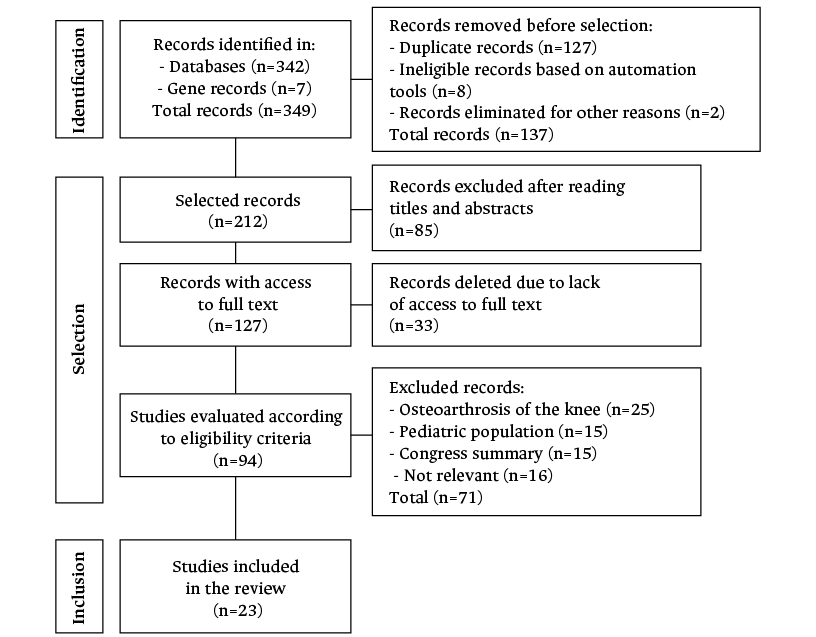
The initial search yielded 349 records, of which 127 were duplicate, 8 were identified as ineligible using the automation tools, and 2 were excluded for other reasons. 85 of the 212 articles were excluded after analyzing the titles and abstracts and 33 were excluded because the full text could not be accessed. Based on the selection criteria, 25 of the 94 articles reviewed were removed because they addressed knee OA, 15 because they were performed in children, 15 because they were conference abstracts, and 16 because they were deemed irrelevant. As a result, the present systematic review comprises 23 papers. Figure 1 depicts the flow chart for the search and selection of articles.
Figure 1. Flowchart of the search and selection of articles.
Source: Own elaboration.
Pathophysiological mechanisms associated with developmental dysplasia of the hip and hip osteoarthrosis
Genetic mechanisms
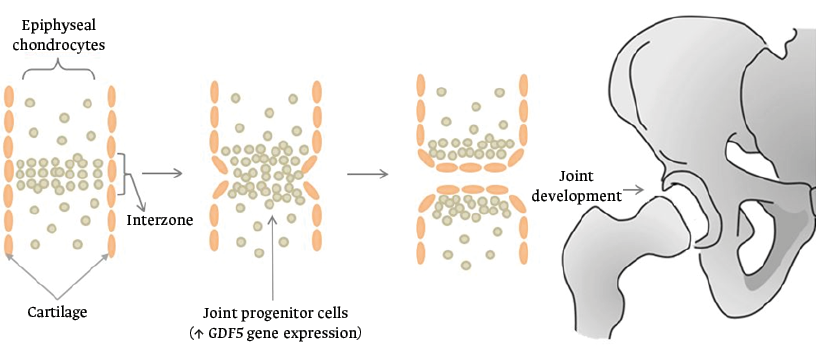
The onset of histogenesis of a joint is preceded by the appearance of a cellular block called interzone, where the future joint is outlined and the expression of new genes occurs, including the GDF5 gene (Figure 2).17
Figure 2. Interzone formation during joint histogenesis.
Source: Own elaboration.
Studies in experimental animals have shown that, in the proximal region of the joint, there are cells expressing the Sox9+, Col2a1+ and Gdf5 genes, which migrate to the joint formation zones to activate Gdf5+ cells. Thus, joint development is considered to be influenced by two biological processes known as the “temporal hypothesis” and the “spatial hypothesis”.17
The temporal hypothesis refers to the expression of GDF5+ cells during early joint development that contribute to the formation of the epiphysis. The spatial hypothesis refers to the fact that the location of these progenitor cells in the interzone determines the ultimate lineage destination.17 Consequently, the constant dynamic flow of GDF5+ cells in the interzone mobilizes the cells in different spatiotemporal patterns. In this sense, this spatiotemporal asymmetry is considered to favor joint development in long bones, such as the humerus and femur, in which it has been demonstrated that there is a higher concentration of GDF5+ cells compared to their joint counterpart.
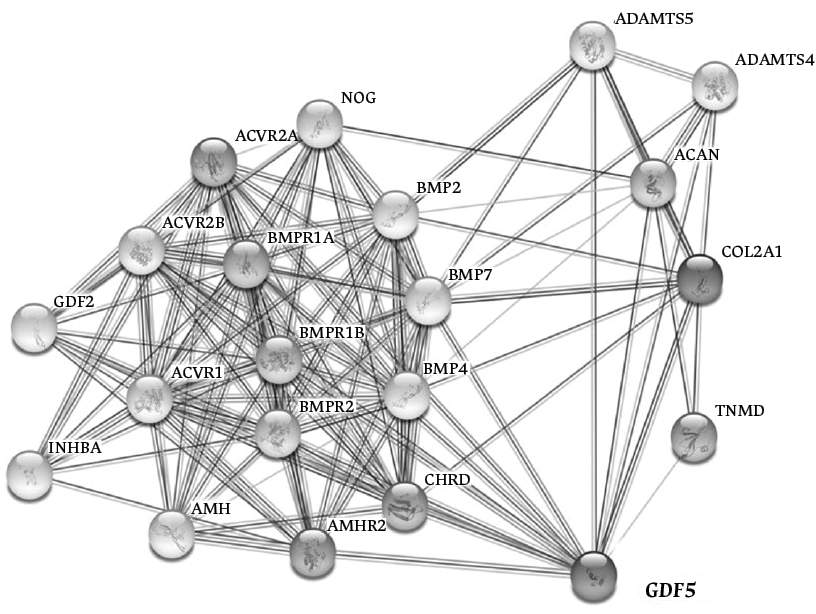
Furthermore, molecular studies have shown that the presence of mutations in the GDF5 gene can affect cartilage and bone development, since GDF5, together with several genes, participates in an interactome (Figure 3), which involves receptors associated with ligands that in turn bind and activate transcriptional regulators (known as SMADs). SMADs contribute to several phenomena related to joint embryogenesis, such as right-left pattern formation, cartilage morphogenesis, inhibition of chondrocyte differentiation, regulation of cell-cell interactions during inflammation and tumorigenesis, and participation in apoptosis pathways, among others.17,19,20
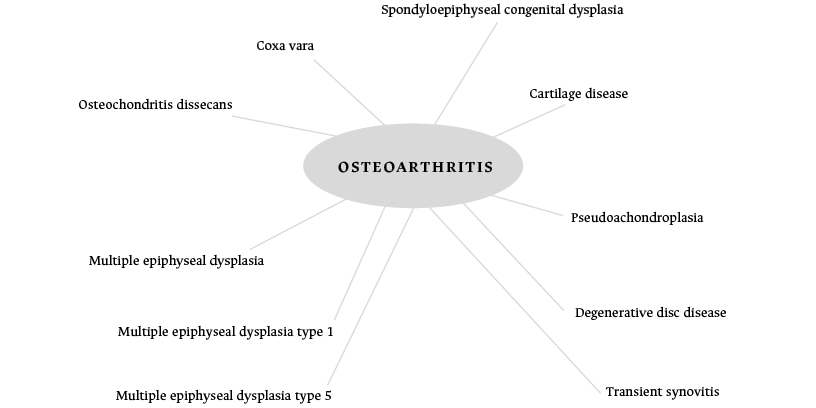
Thus, the interactome is a network of interactions between biomolecules that do not necessarily have the same type or function. Many of these interactomes are related to diseasomes, which are a group of diseases that are interrelated by their genetic origins; in other words, a gene (or group of genes) is connected to a given disease through mutations and/or genetic variants. Considering the above, it could be assumed that DDH and OA could have a shared genetic origin through GDF5, since these two diseases seem to originate from the same functional module through this gene (Figure 4).21,22
Figure 3. GDF5 gene and its participation in an interactome by coexpression, neighborhood, or fusion.
Source: Adapted from the STRING Database.20
Figure 4. Osteoarthritis-related diseases and mutations in the GDF5 gene in the diseasome.
Source: Adapted from Exploring Data.21
Inflammatory mechanisms
In the past, OA was considered to be a non-inflammatory disease because no biochemical data of inflammatory disease were identified in the synovial fluid of patients with OA compared to that of patients with rheumatoid arthritis. However, these theories were eventually revised when it was observed that patients with OA presented synovitis with inflammation similar to that found in patients with a history of trauma. Thus, the inflammatory process is now considered part of the origin and progression of OA.23
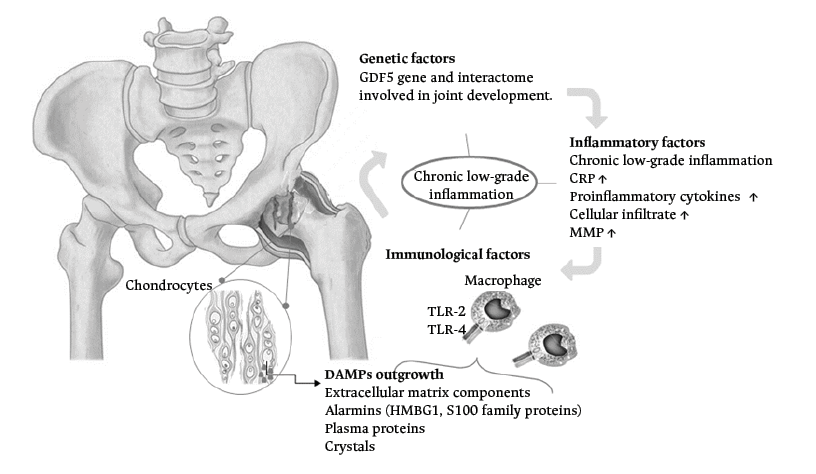
Risk factors for OA include mainly age, obesity, presence of joint trauma, and biomechanical disorders. All these risk factors are considered biological alterations that generate chronic low-grade inflammation and could lead to hip joint destruction (Figure 5).23,24
DAMPs: damage-associated molecular patterns; GDF5: growth/differentiation factor 5; HMGB1: high mobility group box 1 protein; MMP: matrix metalloproteinases; CRP: C-reactive protein; TLR-2 and TLR-4: Toll-like receptors.
Figure 5. Diagram of the pathophysiological mechanisms associated with developmental dysplasia of the hip and osteoarthrosis.
Source: Own elaboration.
It has been noted that patients with OA have elevated C-reactive protein and interleukin-6 (IL-6) serum levels in synovial fluids, even before showing the typical radiographic changes of joint wear, such as cartilage loss, joint space narrowing, and the formation of osteophytes.23,25 It has also been described that, in the early phase of OA, an increased amount of a cellular infiltrate comprising mainly macrophages, mast cells, natural killer (NK) cells and some T and B cells, as well as elevated levels of some inflammatory mediators such as prostaglandins and cytokines, can be seen in a sample of synovial fluid, which is different in the late phase of OA.23 Inflammatory mediators could activate the inflammatory process in a non-traditional way, since articular cartilage is not vascularized or innervated, so chondrocytes succeed in increasing the production of matrix metalloproteinases (MMPs) through such inflammatory signals, degrading the cartilage into small fragments that float in the synovial fluid within the joint and exacerbating inflammation.26
Another pathophysiological mechanism related to the inflammatory process in OA is the overexpression of leptin, which is a hormone synthesized mainly by adipose tissue. It has been described that patients with OA and obesity can overexpress leptin, stimulating an increased expression of interleukin-1 (IL-1), IL-6, MMP9, and MMP13, as well as an increase in NK cytotoxicity and the activation of neutrophils, basophils, and eosinophils.26,27 On the other hand, experimental studies have found that the process of histone acetylation and deacetylation of deoxyribonucleic acid (DNA) of human chondrocytes regulates mainly MMP genes, which contribute to cartilage catabolism under the influence of IL-1, leading to joint damage due to infammation.28 In this sense, it is safe to state that inflammation, specifically low-grade inflammation, has an important influence on the pathogenesis of OA.
Immunological mechanisms
Some organs and cells in the human body are immunologically privileged, meaning that they are able to tolerate the presence of antigens without triggering an inflammatory response. Joint tissue has therefore been described as part of this category.29 Cartilage does not contain blood vessels or a lymphatic or nervous system, so it is considered a basic tissue, as it only consists of chondrocytes, type II collagen, and proteoglycans that are responsible for providing hydraulic resistance to mechanical forces. The absence of vasculature results in the chondrocytes receiving nutrients and eliminating waste material by diffusion through the extracellular matrix to and from the synovial fluid, respectively. Consequently, any type of joint injury, whether congenital or acquired, could hinder cartilage repair, degrade it, and cause OA.30
As discussed in the previous section, inflammatory signals can increase the production of MMPs in the joint and produce small cartilage fragments that float in the synovial fluid, acting as damage-associated molecular patterns (DAMPs). These DAMPs are intracytoplasmic or nuclear molecules released from cells that underwent immunogenic apoptosis, extracellularly exposing their intracellular components and alerting the body to produce an innate immune response.23,26,31 DAMPs associated with joint damage include fibronectin, hyaluronan, alarmins, some calcium phosphate and/or uric acid crystals, plasma proteins, among others (Figure 4).23,26
DAMPs in the joint stimulate the synthesis of proinflammatory cytokines such as IL-1β, IL-6, tumor necrosis factor alpha (TNFα), vascular endothelial growth factor (VEGF), MMP1, and MMP3, causing chondrolysis. Similarly, it has been reported that sites with tissue damage and inflammation may present vascular leakage and exudate of plasma proteins into the synovial fluid, acting as DAMPs since they promote the production of macrophages that bind to pattern recognition receptors, known as Toll-like receptors-2 (TLR-2) and Toll-like receptors-4 (TLR-4), initiating and promoting OA.23,25,26
Complement system activation is another feature of innate immunity that may contribute to cartilage damage, since high concentrations of these proteins have been found in synovial fluid of patients with OA. This has been demonstrated in proteomic studies in which the upregulation of genes encoding complement proteins has been evidenced, along with the downregulation of inhibitory molecules of this same system.23
On the other hand, oxidative stress is another mechanism of innate immunity that can cause chondrocyte senescence. In general, senescence in cell cultures can be replicative or secretory. Replicative senescence refers to a decrease in cell division rate during periods of vigorous proliferation, whereas secretory senescence refers to the fact that cells may have a low capacity for cell division but a high capacity for synthesis of soluble mediators.26 Chondrocytes belong to the latter form of senescence, so it is considered that, as a person ages, their articular chondrocytes fall into secretory senescence, stimulating the production of IL-1β and MMP13, and causing local inflammation and chondrolysis, which can lead to the development of OA in the joint.26
Discussion
As of the time of conducting this study (February 2023) and according to our search for evidence, there are few publications on the genetic, molecular, and experimental factors that may possibly associate DDH with OA, and some of those studies are inconclusive.6,9,14,15 However, in the last decade, gene-gene interactions have become relevant, as they show how different groups of genes can be involved in the same functional pathway to create between pathway modules,32 so that a simple variation of a nucleotide in a gene could alter the pathway. A clear example for this is what has been pointed out in this review regarding the role of the GDF5 gene in chondrogenesis and joint osteogenesis in an active manner. 6,7 Any molecular alteration in this gene could lead to chondrolysis and other consequences in cartilage, bone, and joint development.6,19
However, not only genetic factors are related to DDH and its tendency to predispose to OA. As noted in this article, inflammatory and immunologic factors trigger a process, perhaps originating from the mechanical instability caused by DDH, that results in chronic low-grade inflammation in all joint components, clinically manifesting as fibrosis, articular cartilage degradation, thickening of the subchondral bone, osteophyte formation, synovitis, meniscal and ligament degradation, and capsular hypertrophy.1,6,15,33
Moreover, proteomic studies performed in synovial fluid have shown that there are new biomarkers, such as alpha-1-acid glycoprotein (AGP1), which is currently only evaluated sporadically because it is not a biochemical marker analyzed in routine examinations. AGP1 is involved in both joint inflammatory and immunological pathways in inflammation as well as in immunological processes in the joint. This is relevant for the diagnosis of OA because OA is considered a joint-circumscribed disease, which means that serum levels of proinflammatory markers are lower than markers measured in synovial fluid in these patients.34 In turn, based on blood tests, C-reactive protein is one of the few proteins found to be elevated in patients with OA, which is of special importance since it has been described that there is a correlation between having a high level of this protein and the degree of inflammatory infiltrate in the patient’s joints.34
Therefore, DDH is a disease that should be diagnosed and treated in a timely manner, because it can result in physical sequelae that include movement limitations, limb shortening, claudication, scoliosis, pain, knee deformities, premature wear of the contralateral hip, etcetera.2 Such sequelae can lead to patient disability or the condition can cause complications such as OA, which will eventually require hip arthroplasty, whose risks range from superficial infection to periprosthetic femoral fractures or deep vein thrombosis that require intensive care.2
Therefore, clinical and radiological follow-up is recommended for patients with DDH during the first years of life to assess bone maturation.4 However, it should be kept in mind that it is currently not possible to predict the development of OA based on a history of DDH, even in patients with apparently healthy hips, since they can also develop this condition.4 Recent research has proposed that certain genetic, inflammatory and immunological mechanisms could be responsible for initiating, prolonging and maintaining joint damage in the hip,26 so understanding the pathophysiology of these diseases will favor the implementation of novel treatments to mitigate or resolve joint damage.26 It is recommended to take into consideration serum and synovial fluid biochemical markers for the follow-up of DDH and OA.34
Conclusions
DDH has been considered a purely mechanical disease due to the presence of instability and/or dysplasia of the affected bone structures; however, such instability can generate chronic low-grade inflammation over time and lead to the development of OA in young adulthood.23,26-28
From the information currently available, it can be concluded that there is a very close relationship between DDH and OA occurring at an early age, as the mechanical process of friction between the hip joints and the femoral head generates chronic inflammation through a process that involves the innate and adaptive immune system.14,15 The chronic inflammatory process that unfolds is a response of the body to the damage, through which it tries to repair the structures involved, but the persistence of the mechanical damage produces a permanent activation of the inflammatory systems, which in turn may be the cause of the long-term harmful effects.23,28
Furthermore, the process of chronic inflammation involves the activation of macrophages along with the elevation of proinflammatory cytokines that prolong the damage to the structures, as detrimental elements such as oxygen-derived free radicals are generated, which, added to everything mentioned above, initiate a continuous joint degradation.23,26-28
The pathophysiological mechanisms of the development of DDH and OA are not completely known. Nonetheless, understanding the interactions between genetic, inflammatory and immunological factors will help the treating physician to establish a timely diagnosis and, hence, provide treatment aimed at reducing and, if possible, controlling long-term damage, as well as improving the patient’s quality of life.
Conflicts of interest
None stated by the authors.
Funding
This literature review was financially supported by the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado.
Acknowledgments
The authors would like to express their gratitude to the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado for providing the necessary resources.
References
1.Moraleda L, Albiñana J, Salcedo M, González-Moran G. Displasia del desarrollo de cadera. Rev. Esp Cir Ortop Traumatol. 2013;57(1):67-77. https://doi.org/f2jnrh.
2.Cymet-Ramírez J, Alvarez-Martínez MM, García-Pinto G, Frías-Austria R, Meza-Vernis A, Rosales-Muñoz ME, et al. El diagnóstico oportuno de la displasia de cadera. Enfermedad discapacitante de por vida. Consenso del Colegio Mexicano de Ortopedia y Traumatología. Acta Ortop Mex. 2011;25(5):313-22. https://doi.org/kgrm.
3.Sarmiento-Carrera N, González-Colmenero E, Vázquez-Castelo JL, Concheiro-Guisán A, Couceiro-Naveira E, Fernández-Lorenzo JR. Riesgo de displasia del desarrollo de la cadera en pacientes sometidos a versión cefálica externa. An Pediatr (Barc). 2018;88(3):136-9. https://doi.org/kgrn.
4.Nemeth BA, Narotam V. Developmental dysplasia of the hip. Pediatr Rev. 2012;33(12):553-61. https://doi.org/ddfw4j.
5.Storer SK, Dimaggio J, Skaggs DL. Developmental Dysplasia of the Hip. Am Fam Physician. 2006;74(8):1310-6. https://doi.org/kgrp.
6.Hatzikotoulas K, Roposch A, Shah KM, Clark MJ, Bratherton S, Limbani V, et al.. Genome-wide association study of developmental dysplasia of the hip identifies an association with GDF5. Commun Biol. 2018;1:56. https://doi.org/kgsh.
7.Knut & Alice Wallenberg Foundation. The Human Protein Atlas. Suecia: 2003 [Cited 2021 October 15]. Available from: https://www.proteinatlas.org.
8.Lespasio MJ, Sultan AA, Piuzzi NS, Khlopas A, Husni ME, Muschler GF, et al.. Hip Osteoarthritis: A Primer Perm J. 2018;22:17-84. https://doi.org/gp5nn2.
9.Lievense AM, Bierma-Zeinstra SMA, Verhagen AP, Verhaar JAN, Koes BW. Prognostic factors of progress of hip osteoarthritis: a systematic review. Arthritis Rheum. 2002;47(5):556-62. https://doi.org/cfqrpc.
10.Leung, YY, Pua, YH, Thumboo J. A Perspective on Osteoarthritis Research in Singapore. Proc Singap Healthc. 2013;22(1), 31–9. https://doi.org/kgsj.
11.Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011; 14(2):113-121. https://doi.org/fhggmq.
12.de Andrade DC, Saaibi D, Sarría N, Vainstein N, Cano-Ruiz L, Espinosa R. Assessing the burden of osteoarthritis in Latin America: a rapid evidence assessment. Clin Rheumatol. 2022;41(5):1285-92. https://doi.org/grvf7d.
13.Terjesen T. Residual hip dysplasia as a risk factor for osteoarthritis in 45 years follow-up of late-detected hip dislocation. J Child Orthop. 2011;5(6):425-31. https://doi.org/d4q5wd.
14.Woetzel D, Huber R, Kupfer P, Pohlers D, Pfaff M, Driesch D, et al. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res Ther. 2014;16(2):R84. https://doi.org/gb92fh.
15.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77-94. https://doi.org/f4vp59.
16.Mabuchi A, Nakamura S, Takatori Y, Ikegawa S. Familial osteoarthritis of the hip joint associated with acetabular dysplasia maps to chromosome 13q. Am J Hum Genet. 2006;79(1):163-68. https://doi.org/bwfs27.
17.Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016;15(12):2577-87. https://doi.org/gh9qxr.
18.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/gjkrwz.
19.Degenkolbe E, König J, Zimmer J, Walther M, Reiner C, Nickel J, et al. A GDF5 point mutation strikes twice--causing BDA1 and SYNS2. PLoS Genet. 2013; 9(10):e1003846. https://doi.org/f9snwh.
20.ELIXIR Core Data Resources y Global Biodata Coalition. STRING CONSORTIUM. ELIXIR Core Data Resources y Global Biodata Coalition; 2020. [Cited 2021 December 13]. Available from:: https://string-db.org/.
21.Gómez R. Exploring Data. Gómez R; 2019 [Cited 2021 December 19]. Available from: https://exploring-data.com/vis/human-disease-network/.
22.Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL. The human disease network. Proc Natl Acad Sci U S A. 2007;104(21):8685-90. https://doi.org/bt6qvc.
23.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77-94. https://doi.org/f4vp59.
24.González-Costa M, Padrón-González AA. La inflamación desde una perspectiva inmunológica: desafío a la Medicina en el siglo XXI. Rev Haban Cienc Méd. 2018;18(1):30-44.
25.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21(1):10-5. https://doi.org/f2m64g.
26.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21. https://doi.org/f2m6p7.
27.Zeddou M. Osteoarthritis Is a Low-Grade Inflammatory Disease: Obesity’s Involvement and Herbal Treatment. Evid Based Complement Alternat Med. 2019;2019:2037484. https://doi.org/kgsp.
28.Rogers EL, Reynard LN, Loughlin J. The role of inflammation-related genes in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1933-38. https://doi.org/f79jcg.
29.Fujihara Y, Takato T, Hoshi K. Macrophage-inducing FasL on chondrocytes forms immune privilege in cartilage tissue engineering, enhancing in vivo regeneration. Stem Cells. 2014;32(5):1208-19. https://doi.org/gr9c54.
30.Abazari A, Jomha NM, Elliott JAW, McGann LE. Cryopreservation of articular cartilage. Cryobiology. 2013;66(3):201-9. https://doi.org/f4vhs3.
31.Rojo-León V, Aguilar-Cázares D, Prado-García H, Carlos-Reyes Á, López-González JS. Participación de los patrones moleculares asociados al daño en el tratamiento convencional del cáncer. Rev Invest Clin. 2012;64(3):284-93.
32.Costanzo M, Kuzmin E, van Leeuwen J, Mair B, Moffat J, Boone C, et al. Global Genetic Networks and the Genotype-to-Phenotype Relationship. Cell. 2019;177(1):85-100. https://doi.org/gfw9gq.
33.Roh JS, Sohn DH. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018;18(4):e27. https://doi.org/gd6rcx.
34.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. https://doi.org/fxrvc8.
 Liliana García-Ortiz2
Liliana García-Ortiz2 José Gutiérrez-Salinas3
José Gutiérrez-Salinas3 Tania Jazmín Ruiz-Sánchez4
Tania Jazmín Ruiz-Sánchez4